Please select your Region.
Please select your Region.
Aug 27, 2012
Research into personalized medicine (in which the determination of the individual’s genotype forms the basis for optimum drug selection/ dosage according to that person’s body and the disease state) is progressing at a very fast pace.
However, this kind of pre-treatment diagnosis has typically been very difficult-to-perform, requiring a high degree of operational skill and a long period of time until results become available.
In 2009, ARKRAY released the i-densy™ IS-5310, the world’s first fully automatic and desktop sized SNP*1 analyzer for research use. This analyzer is used for the fully-automatic detection of genetic base pair sequences relating to the metabolism of drugs, as well as genetic mutations associated with cancer. ARKRAY is moving forward with research mainly in the fields of drug efficacy and side-effects associated with anti-cancer treatments, and continues to support research facilities around the country.
The medical-use gene analyzer i-densy™ IS-5320 is an improvement on and successor to the fully-automatic SNP analyzer i-densy™ IS-5310 (for research use).
Compatible in vitro diagnostic reagents were developed in parallel (with instrument development) for use on the i-densy™ IS-5320, allowing simple analysis of base pairs which in turn enables prediction of the effect of administered drugs as well as offering complementary diagnoses. We fully expect this system to contribute to speedy decision making, reductions in the rate of side effects and cost savings in the medical field.
○Fully-automatic measurement
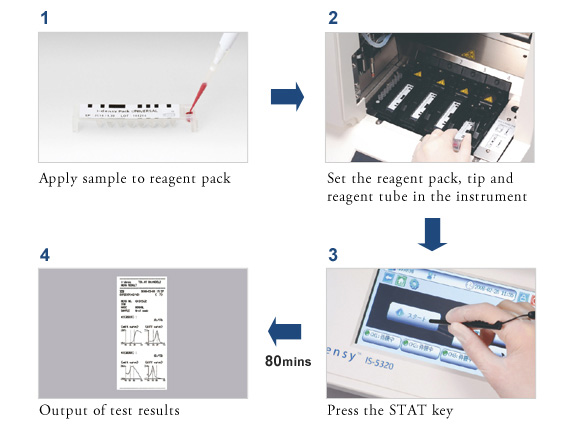
Simple measurement possible with no special skills required- just set the sample (blood or oral swab)
Fully automatic in a single device: From DNA extraction from sample to gene amplification and on to gene type determination
○High speed measurement
Gene typing which previously took days from sample collection to output of results, now takes just 80 minutes

1) SNPs (Snips)
SNPs, also known as Single Nucleotide Polymorphisms: Minute changes occur in certain genes and when occurring in 1% or more of genetic base pairs, they are referred to as SNPs. Analysis of SNPs and their connection to drug efficacy and side effects, allows for selection of the most-appropriate treatment and dosage, as well as offering high hopes for personalized medicine in the medical field.
Set the sample (blood or oral swab) in the pack, set the pack in the instrument and press the start button for rapid gene analysis.
Name |
Gene Analyzer i-Densy IS - 5320 |
|---|---|
Release date |
28 August 2012 (Tues) |
Specifications |
|
Meas. sample type | Biological samples such as whole blood, purified nucleic acids |
Measurement principle | Nucleic acid amplification + Tm analysis |
Processing speed | 80mins/ reagent pack *may vary depending on measurement item |
Minimum sample volume | 70µL of biological sample (whole blood etc), or 4µL of purified nucleic acid |
No. of set samples | 1-2 samples |
Memory | 100 measurements/ user (A maximum of 25 users may be registered) |
Power supply | AC100, less than 300VA |
Outer dimensions | 410mm (Width) X 450mm (Depth) X 415mm (Height) |
Weight | 27kg |
Medical device filing no. |
25B1X00001000018 |
Class categorization |
Class I (General Medical Device)/ medical device with special maintenance controls |
This product is available through ARKRAY, Marketing, Inc.; a member of the ARKRAY Group responsible for sales within Japanese.
![]()