Please select your Region.
Please select your Region.
Dec 17, 2014
The influenza virus spreads predominantly in the winter season of each year, and many people suffer from this acute viral infection.
Serious cases of the illness carry the risk of pneumonia in the elderly, and may cause complications of encephalitis and encephalopathy in small children. To prevent complications and serious incidents of the infection, diagnosis and treatment must be commenced at an early stage and the rapid testing of the influenza virus at the treatment site plays an important role.
SPOTCHEM i-Line FluAB-S released by ARKRAY Inc. (hereafter, ARKRAY) is a kit that detects Type A and Type B influenza virus antigens in fluids obtained by nasal swabs or nasal aspiration. Results can be determined visually as well as by using the dedicated measurement device (densitometry analysis device, SPOTCHEM IL SL-4720), and both positive and negative results can be determined in roughly 10 minutes (a 5 minute reduction compared to existing products*.) Through rapid testing, waiting time will be shortened, reducing the burden on the patient and improving treatment efficiency, leading to reduced risk of contagion for the flu virus.
ARKRAY aims to continue ongoing development of immediate testing devices and kits to facilitate rapid diagnosis and appropriate treatment in the front line of the medical field.
* Comparison to ARKRAY's existing product, SPOTCHEM i-Line FluAB
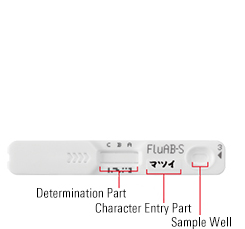
Testing Steps
Measurement process for the device (using the dedicated measurement device, SPOTCHEM IL SL-4720)

Reference Materials
ARKRAY has been selling the SPOTCHEM series of simple testing devices for point of care (POC) testing in clinics since 1988.
The SPOTCHEM IL SL-4720 is a small rapid testing device that automates immunochromatic testing.
This device was developed from the concept of improving treatment quality by using a device that conducts tests quicker, more accurately and easily than the previous practice of visual inspection.
By entering the patient name in the reagent cartridge for the device, patient names will be printed out with the measurement results, reducing the risk of mistaking specimens.
Also, the installation area of the device is less than the size of an A4 sheet of paper, so it does not take up much space.

Measurement using the SPOTCHEM IL SL-4720
| Name | Influenza Virus Kit SPOTCHEM™ i-Line FluAB-S |
|---|---|
| Release Date | December 22, 2014 (Monday) |
| Specifications | |
| Measurement Target | Fluids sampled by nasal swab or nasal aspiration |
| Measurement Items | Influenza virus types A and B |
| Measurement Device | Densitometry analysis device SPOTCHEM IL SL-4720 |
| Measurement Principle | Immunochromatographic method |
| Measurement Time | Approx. 10 minutes per specimen |
| Storage Method | 2 to 30 C(do not freeze), 18 months |
| Packaging Unit | 10 units (10 tests) Test cartridges, specimen extraction fluid (extraction tubes), filter nozzles |
| Retail Price | Preferred Delivery Price: 12,000 Yen (before tax) |
| Approval No. | 22600AMX01321000 |
| Product Category | Extracorporeal Diagnostic Agent |
| Classification | Class III |
This product will be sold through ARKRAY Marketing, Inc. (ARKRAY, Inc.'s distributor in Japan).
This product is only available in Japan and is not sold in other countries.
![]()